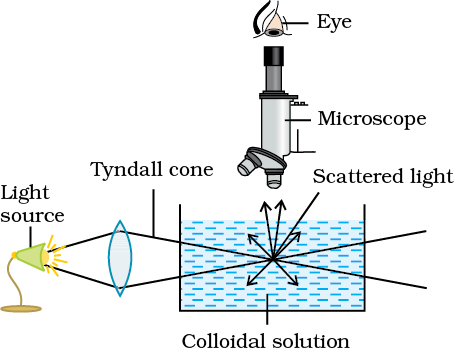
When a beam of light passes through a colloidal solution, the path of the beam of light gets illuminated due to the scattering of light by colloidal particles. This is the phenomenon known as the Tyndall Effect. Initially observed by Faraday, it was studied in detail by John Tyndall, who gave the name to this phenomenon

Some terms above might sound foreign such as ‘colloidal’ and ‘scattering’. Let’s understand some basic concepts before we delve into understanding what actually is happening
Index
Types of Solute-Solvent Mixtures
Typically a solute-solvent mixture will involve addition of the solute which could be in any phase (solid, liquid, or gas) to the solvent which again could also be in any phase. Usually the solute is lesser in volume as compared to the solvent. We can divide the types of solute-solvent mixtures based on the size of the particles of the solute into three categories, namely:
- True Solutions: Here, the particles of the solute have a diameter less than 1 nm.
- Colloids: The diameter of particles in colloids typically ranges between 1nm and 1000nm.
- Suspensions: Such mixtures have particles with diameters greater than 1000 nm.
Such a division holds merit as these mixtures have properties that differ. It must be noted these demarcations are but ranges, as very fine suspensions can show the Tyndall effect, a property that is typical to colloids. Here, particles do not necessarily refer to atoms, molecules and can refer to aggregations of the substance that they exist in the mixture. Particles might also refer to aggregations or solitary macromolecules like starch, cellulose, etc.
Colloidal mixtures form a large part of life. Paint, gem-stones, smoke, dust, fog, are some examples. The food we eat like milk and butter, to the blood that runs in our veins, are all colloids.
Scattering of Light by Particles
The Tyndall effect is caused by the scattering of light by particles. But what is scattering of light? It is the property of particles to interact with light.
When light hits a particle, there are three events, as-
1. The particle absorbs some energy of the light.
2. The particle emits some of the energy of the incident light in the form of light radiation in all directions.
3. Part of the incident light is unaffected and proceeds on its original straight-line path.
There are some theories developed to explain the scattering of light. The most well-known scattering of light is Rayleigh scattering, something which is responsible for the blue color of the sky. Under Rayleigh approximations which imply that the particle size < 1 /10 of the wavelength of light, we can explain scattering.
Light is an electromagnetic wave, and hence are essentially oscillations in the electric and magnetic field. These oscillations influence the electron clouds of the atoms or molecules and create oscillations in the atoms electron cloud. These electron cloud oscillations are disturbances in the electric and magnetic field and hence result in radiation of energy in the form of electromagnetic waves.
The above explanation is but a crude approximation of what actually occurs, and the inner workings, and the physics involved albeit classical are out of the scope of this discussion.
Rayleigh Scattering is a special case of scattering, with its justified explanation and results. One of the important results from Rayleigh Scattering is that the amount of scattering is inversely proportional to λ4 (λ being the wavelength of the incident light). This means that shorter (blue) wavelengths are scattered more strongly than longer (red) wavelengths. This forms the reason(in part) as to why the sky is blue. Tyndall effect too has scattering which is seen to follow the above result. Scattering of light also has a direct correlation with the size of the particle. As the size of the particle increases the amount of scattering increases.
Understanding the Tyndall Effect
The Tyndall effect is a result of the scattering of light incident on the colloidal particles. The particle interacts with the incident light. It ends up emitting light in all directions and is illuminated. A part of light proceeds unaffected until it interacts with another particle which then illuminates. The process keeps repeating, and all particles that lie on the path of the light are illuminated hence showing the path of travel of the beam of light.
Why don’t true solutions show the Tyndall effect?
The size of the particles in a solution is too small to bring about sufficient scattering to illuminate themselves, and hence show the path of the light.
Why don’t suspensions show Tyndall effect?
While certain very fine suspensions with particular sizes closer to the particular range of colloidal particles show Tyndall effects, most suspensions don’t. The scattering becomes too much as the size of particles is bigger than colloidal particles. This results in a very diffused beam that is not concentrated, and hence one has no idea what path the light takes. If it’s a very heavy suspension, the solute might precipitate and there might not even be particles in the line of the path of light to bring about scattering.
Try it out yourself!
It is quite feasible to try and observe the Tyndall Effect by doing this simple experiment. You only need 2 glasses made up of glass, some water, sugar (or salt), and some milk. Follow the steps below:-
- Step 1: Prepare some sugar or salt solution by adding some sugar/salt to the water and mixing it thoroughly. Ensure that there is no undissolved sugar/salt.
- Step 2: Separate the solution into two glasses (make sure that the glasses are made of glass). Place the glasses next to one another.
- Step 3: Add a teaspoon or 2 of milk to one of the glasses and mix thoroughly by stirring with a spoon. Do this until you are sure that the milk has mixed evenly throughout the solution.
- Step 4: Using a torch or any source of light, aim a beam light such that it passes through both the glasses.
- Step 5: Observe the Tyndall effect in the glass with milk added to it. You won’t see Tyndall effect in the plain salt/sugar solution.
Note: It is possible that there might be a beam of light visible in the plain sugar/salt solution. This is the result of some impurities like dust, etc. that were already present in the glass or were part of the added sugar and salt. These impurities are big particles and hence show scattering at visible levels.

