Clean and pure energy is something that we are in dire need. Hydrogen is one such sustainable source of energy that avoids toxic emissions and adds a lot of value to sectors in the economy including transportation, power generation, etc. Storing hydrogen and transporting it is heavy on expenses and is vulnerable to contamination. Thus, having a technology that helps make this process of storing and transporting hydrogen need is essential.
Researchers are searching for other more efficient methods which reduce the costs and are simple. Increasing efficiency means that there will be more benefits in applications like vehicle industries, portable power, etc.
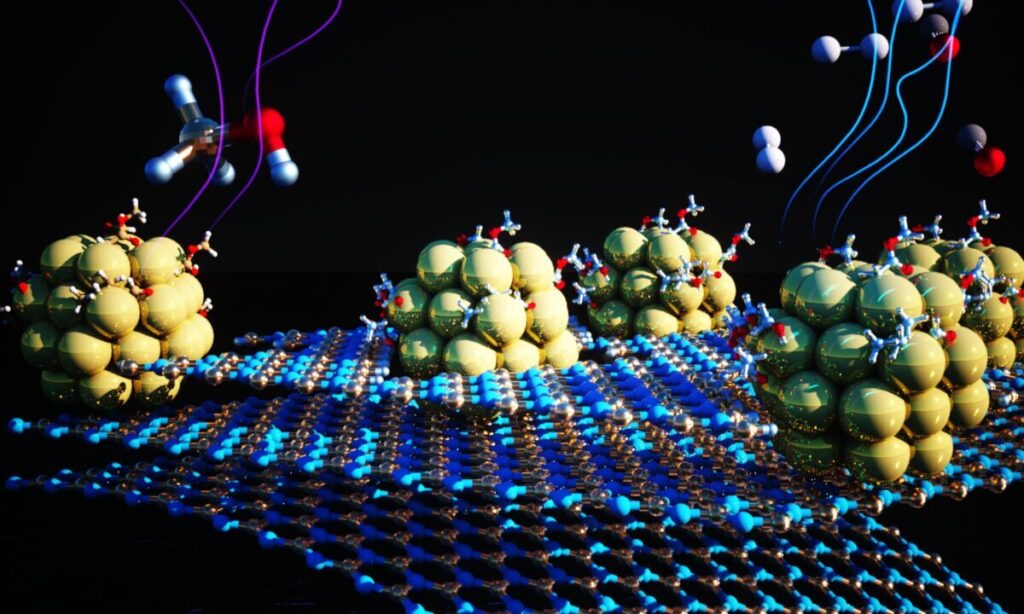
As reported in journal Proceedings of the National Academy of Sciences, researchers have found an effective material for extracting hydrogen from alcohols. The material which is a catalyst is made from tiny clusters of nickel-metal anchored on a 2-D substrate.
The research led by Lawrence Berkeley National Laboratory’s (Berkeley Lab) Molecular Foundry found that the catalyst could cleanly and efficiently speed up the reaction that removes hydrogen atoms from a liquid chemical carrier.
Rather than using existing options which include making the material from precious metals, the new technique includes material from earth-abundant materials and this material help to make hydrogen a viable source of energy.
Jeff Urban, the Inorganic Nanostructures Facility director at the Molecular Foundry who led the work, said that this was not merely a catalyst with higher activity, but it was also a broader strategy for using affordable metals in a broad range of reactions.
The research is part of the Hydrogen Materials Advanced Research Consortium (HyMARC).
The catalysts used not only to increase the rate of a chemical reaction but they also could hold a particular molecule stable or could be used for other purposes. Usually, the catalysts used for producing hydrogen are made from precious metals, they cost high and are low in abundance. They are also susceptible to contamination. On the contrary, less expensive catalysts made from less expensive metals are less effective and less stable, thus limiting their activity.
Urban and his team changed a strategy that focuses on tiny, uniform clusters of nickel-metal which maximize the exposure of reactive surface, thus improving the performance and stability. The drawback with this is that they tend to clump together, thus inhibiting their reactivity.
Postdoctoral research assistant Zhuolei Zhang and project scientist Ji Su, designed and performed an experiment that combatted clumping by depositing 1.5-nanometer-diameter nickel clusters onto a 2-D substrate made of boron and nitrogen engineered to host a grid of atomic-scale dimples. This made the nickel clusters become evenly dispersed and securely anchored in the dimples. As a result, thermal and chemical properties improved greatly. It also prevented clumping.
Urban added that this has paved a way for understanding many things in other processes.
Detailed X-ray and spectroscopy measurements, combined with theoretical calculations, revealed much about the underlying surfaces and their role in catalysis. Using advanced tools, the researchers found that there were changes in the physical and chemical properties of the 2-D sheets while tiny nickel clusters formed and deposited on them.
Berkeley lab team will further work on the strategy of modifying 2-D substrates in ways that support tiny metal clusters, to develop even more efficient catalysts. The technique could help to optimize extracting hydrogen from liquid chemical carriers.
Journal Reference:
Zhuolei Zhang et al, Enhanced and stabilized hydrogen production from methanol by ultrasmall Ni nanoclusters immobilized on defect-rich h-BN nanosheets, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.2015897117