Imagine this. It is the 16th of July, 1945. You are in a desert in New Mexico early in the morning. In a short while, history will be made here.
After the start of the Second World War, in the late 1930s, Otto Hahn had discovered nuclear fission in Germany. Basically, he had observed uranium atoms split into barium. He was an exceptional chemist, and he was absolutely sure about this change. He shared his discovery with his colleague Lise Meitner, who had fled Germany due to Nazi persecution; After all, she was a Jew.
Although initially skeptical, Meitner managed to convince Hahn that it was indeed possible and that a fission reaction had definitely taken place. They published their findings and the shockwave of that bombshell was felt all over the world.
Back in America, Leo Szilárd, a physicist of Hungarian origin, realized the potential of this discovery. The figment of imagination, created by HG Wells seemed possible, neutron bombardment of uranium could be a feasible mechanism, the terrorizing atom bomb was possible! The Nazis, then known for trying to weaponize anything they could, would surely not let this go to waste, would they? Szilard convinced Einstein that this threat was astonishingly close to being realized. They wrote a joint letter to the then President of the United States, FD Roosevelt, addressing this issue. This led to the subject of this text, The Manhattan Project, and the science and story behind it.
Index
Hahn’s Discovery
Before we get into Hahn’s work, let us refresh our ideas of nuclear fission and radiation a little.

Nuclear fission, often called radioactive decay, is a nuclear reaction that involves the splitting of the nucleus of an atom into smaller – lighter – daughter nuclei. A schematic diagram of induced fission, that is, fission that is forced by external stimuli, is given below.
Radiation is simply the release of energy from a body. It can be done using both particles and waves. Certain forms of radiation have enough energy to ionise an atom, and hence they are called ionising radiation. Neutron radiation, or neutron bombardment, has often been used to probe the nucleus. Its uncharged nature makes it very handy as a tool to get close to the highly charged mass present at the centre of an atom.
Now that we have a basic idea of the two concepts, let us move on to Hahn’s experiment.

Hahn had heard that Enrico Fermi was successful in creating transUranium elements, that is, elements heavier than uranium. Curious, Hahn started bombarding all elements with slow-moving neutrons. When he did this with almost all elements, they either turned into a different isotope of the same element, or they decayed into an element that was its neighbor in the periodic table. These were consistent with neutron capture. But something different happened with uranium. It turned into barium and krypton isotopes.

There was something even weirder going on. Mass conservation was seemingly broken!
The sum of the masses of the daughter nuclei was less than the mass of the uranium nucleus. Some of the mass had seemingly vanished into thin air! This is where Einstein’s most famous equation comes into play.
E = mc2
At this point, it seems like everyone and their pet dog is familiar with this equation.
It says that energy and mass are interconvertible by a factor of the square of the speed of light, and the speed of light is approximately 300,000,000m/s! This means that a small amount of mass can release a lot of energy. The implication of this is manifold. If a chain reaction could be induced in a chunk of uranium the size of a basketball, such that all of it undergoes fission, the amount of energy released by it would be more than enough to level a city.
Perhaps Hahn didn’t realize the implication of his work. Perhaps he didn’t believe that nuclear weapons would be invented as fast as they were. Either way, he moved on to do more research into radiochemistry. But unbeknownst to him, he had pushed the first domino that set the stage for the end of World War II.
The Manhattan Project starts
When the news of successful fission by the German side reached the Allies, they became paranoid. This was war after all, and the possibility that the Germans could successfully make a weapon and then use it was hanging over their heads. Thus, after a little coaxing, America started working on nuclear science, with support from the UK and Canada.
The project was called the Manhattan Project as the army support assigned to this project was placed in the Manhattan district. The scientific work was done in multiple places, including in different countries, as a part of the Allied war effort. Here, we will look at two major scientific achievements of this project.
Uranium Enrichment
As discussed here, not all uranium is fissile. There are specific isotopes of uranium that will fission when hit by a neutron, and U235 is one of them. But natural uranium is a mixture, with the majority composition being of U238. Thus, it becomes necessary to increase the concentration of U235 in the sample. This is done by the process of enrichment.

There are multiple ways of enriching uranium, but we will focus on the one that was used during this project.
Remember that isotopes are the same element. They differ only in mass number, not in atomic number. Chemically, they’re identical. Therefore, we must rely on physical methods of separation, and exploit the difference in their mass.
In this case, the separation was done by gaseous diffusion. But wait, I hear you ask, uranium as a gas? Well, uranium is converted to a hexafluoride, UF6. This compound is volatile and vapourises at around 60℃. This makes it slightly easier to work with.
Now let’s talk about gaseous diffusion. This technique is based on Graham’s law, which states that the rate of effusion of a compound, that is, the movement of a particle of a gas through a small hole in the container, is inversely proportional to the square root of the mass of the particle. Expressed mathematically, it becomes
Rate ∝ 1/√Mass
The mass of 235UF6 is 349.034348 g/mol while that of 238UF6 is 352.041206 g/mol. When the numbers are plugged in, it becomes
Rate(235)/Rate(238)= √(352.041206/349.034348) = 1.004298
That is, there is a difference of 0.42% in the rate of effusion for the two species. When done in multiple steps, high enrichment, enough for weapons-grade uranium, was achieved.
This was developed by Francis Simon and Nicholas Kurti at the Clarendon Laboratory in 1940, tasked by the MAUD committee as a part of the British effort in finding out if an atomic bomb was feasible or not.
CP-I
The first major technical achievement of the Manhattan Project was a sustained nuclear fission reaction. Before we go into how it was achieved, let us discuss what it is, and why it was such a big deal.
First, let us go into a nuclear chain reaction. We have already talked of a nuclear reaction. As a recap, when a neutron hits a Uranium – 235 nuclei, fission takes place. The resulting products are daughter nuclei, energy, and two or three neutrons. In a chain reaction, those neutrons go on to bombard more uranium nuclei.
This is an exponential reaction. Let us assume that each fission reaction produces two neutrons. They go on to fission two nuclei, producing four neutrons, which result in eight more neutrons, and so on and so forth.
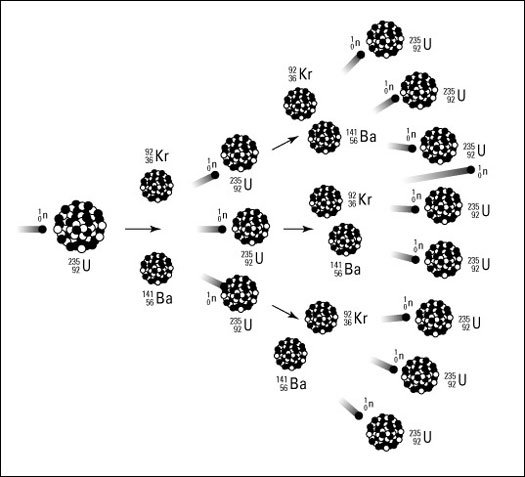
Let me give you an idea of how fast all of this happens. Let’s consider a ball of U235 around 0.1 m in diameter. A neutron travels at around 3% the speed of light, roughly 10,000,000 m/s. Thus, the time taken for a neutron to go through this sphere is:
T = 0.1/10000000 = 10-8seconds
Accounting for the time taken for the uncontrolled fission reaction to occur, that is, for a bomb explosion, the time taken is 80 times this number, that is,
80×10-8 = 8×10-7seconds
This means that the reaction will complete in less than a microsecond.
Now that we have an idea of a chain reaction, let us move to Chicago.
It is December the 2nd, 1942, a few years after Otto Hahn discovered the fission reaction. Enrico Fermi and his colleagues including Szilard were working on fission reactions, especially if a sustained chain reaction was actually possible. Theoretically, if there were enough neutrons, a chain reaction could be sustained. They had constructed a reactor, which was called Chicago Pile I or CP-I.
One by one, they slowly lifted the control rods.

The experiment was a success, and the reaction went critical, indicating that an artificially sustained chain reaction was indeed possible! This was the first nuclear reactor ever made. Less than five minutes later, Fermi ordered the reaction to be shut down. The reactor was disassembled and then remade with concrete shielding.

At that time, it was a moment to celebrate, but the immediate future would not be as pleasant.
And now…
We return to the 16th of July, 1945. Robert Oppenheimer has been heading Project-Y, the part of the Manhattan Project tasked with building the bomb. They were at the Jordana del Muerto (Translated from Spanish: Journey of the dead man. Fitting, don’t you agree?), a desert in New Mexico, waiting to find out if a bomb would work. Most of the work had been done at Los Alamos, everyone was under immense pressure to make this work. After years of development and study, the trigger was pulled.
It was an emotionally charged moment. The potential for destruction had been more than what they ever thought would be possible. Quoting Oppenheimer here, “We knew the world would not be the same. A few people laughed, a few people cried. Most people were silent. I remembered the line from the Hindu scripture, the Bhagavad Gita; Vishnu is trying to persuade the Prince that he should do his duty and, to impress him, takes on his multi-armed form and says, ‘Now I have become Death, the destroyer of worlds.’ I suppose we all thought that, one way or another.”
The world did change from that moment on. Less than a month later, on the 6th of August, 1945, the uranium-based “Little Boy” was dropped on the Japanese city of Hiroshima. A couple of days later, on the 9th, plutonium-based “Fat Man” was dropped on Nagasaki. The estimated loss of life from both these bombings ranges from 129,000 to 226,000, the only thing remaining of the people’s existence being permanent shadows where they were standing when the bombing took place. Many people who escaped the initial explosion suffered from radiation poisoning due to the neutron radiation released from the blast. This marked the end of the Japanese in WWII, and they surrendered.

For all the paranoia about the Nazis getting their hands on the nuclear weapons, there was little to no progress beyond Otto Hahn’s discovery. Hahn himself was horrified at the destruction Fat Man and Little Boy had unleashed, and how the first domino to fall had been his discovery. He became extremely depressed and suicidal and blamed all the deaths on himself. Fortunately, he recovered.
The origin of nuclear sciences was during a time of war, and therefore, the science behind this was harnessed to damage. But times have changed, and with it, the need of the hour. Nuclear sciences have gone on to save lives and have found multiple applications, especially in the fields of energy and medicine. But that does not mean that we ignore the potential for damage. Keep in mind, science is a tool, and the purpose and methodology of the user determine the outcome; good or bad. Let us keep this in mind and move forward towards advancement and progress, while at the same time remember the past of death and destruction.


Pingback: Nuclear Energy: The Benevolent Cousin of the Bomb | AtomsTalk