Ever since the discovery of the nuclear fission of Uranium by Otto Hahn and Lise Meitner in the late 1930s, it has been associated with weaponry. Unfortunately, due to this connection, nuclear energy has been viewed with fear and hostility. In my opinion, this is rather unfair. While it is true that the origin of nuclear technology was during one of the darkest times in world history, we have to remember that one of the earliest breakthroughs in the field was the successful test of the Chicago Pile 1 reactor by Enrico Fermi.
It’s no secret that the same technology that is used to make bombs is used in the reactor for energy production. But how is it different, and how does it work? And how safe is it? We’ll find out here.
Index
Energy in the Nucleus?

The first thing that needs to be addressed is the source of energy in the nucleus. Let us start by defining energy. Here, when I’m talking about energy, I’m referring to the property of matter and radiation which manifests as a capacity to do work (such as causing motion or the interaction of molecules).
To understand where the energy in the nucleus comes from, a couple of topics need to be addressed first.
Conservation of Mass-Energy
When we talk about energy, one of the first properties mentioned is that energy is always conserved in an isolated system. This implies that the amount of energy present in these systems is fixed; it can neither be created nor destroyed. It can only be converted from one form or another.
After the advent of Einstein and his famous equation E= mc2, the law was tweaked to say that mass and energy are interconvertible, and that mass-energy is conserved in a system.

A Nuclear Reaction Explained
Within nuclear physics, a nuclear reaction is one where two atomic nuclei or an atomic nucleus and other subatomic particles collide to give one or more nuclei. Basically, any reaction that involves the nucleus of an atom is called a nuclear reaction. Like any chemical reaction, this process can be either endothermic, where energy is absorbed from the surrounding, or exothermic, where energy is released to the surrounding.

Similar to the reactions in chemistry, there are many kinds of nuclear reactions. The one we require here is the nuclear fission reaction. This reaction is one where a big nucleus breaks into two or more smaller nuclei. Fission reactions can occur naturally; this is what makes certain unstable isotopes of elements radioactive. It can also be induced; this is usually done by bombarding the target nucleus with neutrons. Depending on the target nuclei and the mode of decay, induced fission can have multiple products.

The Role of Binding Energy
Of course, a nucleus needs some energy to cohere. This energy is referred to as binding energy.
To understand this, let us take a look at a curious phenomenon that happens in a nucleus. As an example, consider the atom of Uranium 235.
Logically, it makes sense that the total mass of the atom should be the sum of the masses of its components, right? But the weighing scales say otherwise. We know the mass of a proton, neutron, and electron. When the mass of 92 protons, 92 electrons, and 143 neutrons are added, we get the theoretical mass of an atom of U92235to be 236.9591 atomic mass units (amu). But, we know from experiments that the mass of a U92235atom is 235.0439 amu. Where did the extra mass go? This difference in mass is called the Mass Defect, and this ties in with the concept of binding energy.
It is this difference in mass that is the source of the binding energy in the nuclei. Remember Einstein’s mass-energy equation? Well, this extra mass converted to energy is what is holding the nucleus together. Thus, if you break apart the nucleus into smaller ones, you can harvest the binding energy. This is the basic idea behind almost all nuclear technology involving fission.

Okay, so how do we harvest this energy?
Now that we have a theoretical idea of how to harvest energy from the nucleus, let’s see how it is done in real life. Like almost all other forms of energy generation, the aim is to get a turbine to spin.
This is a schematic of a nuclear reactor:
![A Pressurised Water Reactor [PWR]](https://atomstalk.com/wp-content/uploads/2020/07/A-Pressurised-Water-Reactor.gif)
To put it simply, the heat released during fission reactions is used to convert water into steam. This steam is used to rotate the turbine, which is attached to a generator. When the turbine rotates, the generator produces electricity. The steam is then passed through a condenser, which converts it to water again, and the cycle repeats. This is how we get the energy out of the nucleus of an atom.
Now that we have a rough idea of how things take place, let us look at a few aspects more closely.
The Fuel
You can’t simply stuff a nuclear reactor core with any random isotope and expect to obtain energy. There are certain conditions that determine if an isotope of an element can be used as nuclear fuel. The most important one is that the nuclide must be fissile. This means that the nuclide must be able to capture thermal neutrons and undergo fission. The most common fuel used is Uranium-235.

There are a couple of reasons for this choice. The first and most obvious reason is that U235 is a fissile material. It can be easily split when bombarded with neutrons. Apart from that, Natural Uranium [which is not the same as U235] is found in nature in many parts of the world and is thus easier to procure. The enrichment process, which is done to increase the concentration of fissile uranium in the fuel, is also made easy as we can convert uranium metal into a volatile compound under relatively simple conditions.
Even after enrichment, Uranium has to be processed into fuel rods to be able to be used in the reactor. The series of all processes that describe uranium throughout its life cycle; from mining to processing to generating electricity and finally to its reprocessing and waste is called the Nuclear Fuel Cycle.

Another good fuel used in nuclear reactors is Plutonium-239. This is obtained from the breeding of fertile Uranium-238 in reactors. There is also research into using Thorium-232 as a fertile fuel source. Based on this approach, India’s three-step nuclear program was designed.

Control Rods
The fission reaction, especially in U235, can produce either 2 or 3 neutrons as the products. These products can go and initiate more fission, releasing even more neutrons, which causes more fission, and on it goes. This is a chain reaction. Based on if we control this chain reaction or not, we can get either a reactor or a bomb.

To prevent our source of energy from annihilating us all, we need to control this chain reaction. This is done by removing the extra neutrons from the reaction. This is where control rods come into the picture.
Control rods are rods made of certain elements that are good at absorbing neutrons. They are inserted into the core of the reactor to keep a check on the neutrons that are available for a fission reaction. Of course, the composition of a control rod is important. Depending on the type of reactor, they can be made of chemical elements such as boron, cadmium, silver, or indium. The speciality of these elements is that they are capable of absorbing many neutrons without themselves fissioning.
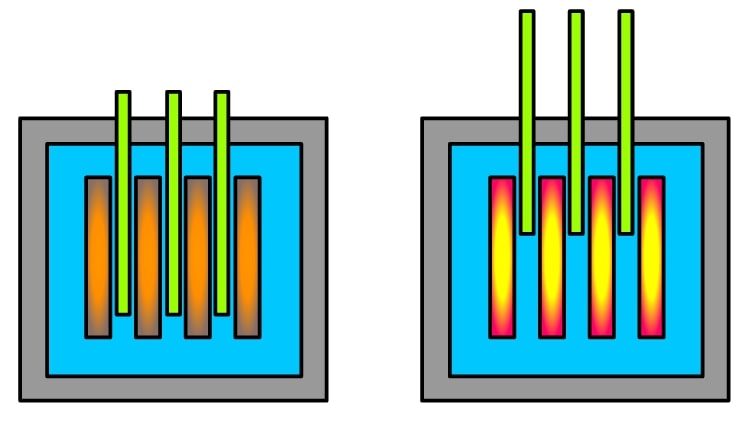
Basically, think of these rods like a switch. When you want the reactor to be switched on, they can be lifted up. When they are lowered, the reactor is switched off.
Moderators
In a nuclear reactor, the neutron has to be at a specific speed to actually initiate fission. To use an analogy, imagine your friend passing by you. If your friend is moving too fast, perhaps on a vehicle, there will be very little interaction between you. The logic is similar here. If the neutrons have too much energy, they don’t cause fission. Thus, it is important to slow down neutrons. This is the job of the moderator.

A good moderator has to slow down the neutrons without absorbing them. Through collisions, the energy of the neutrons has to be reduced. An important parameter to consider here is the size of the moderator molecule. We know from the conservation of momentum that the two colliding particles need to have a similar mass for velocity transfer from the neutron to take place.
Water, or more specifically, the hydrogen atom is an amazing moderator due to the similarity in size. Most reactors use water as a moderator due to its easy availability. Water, specifically H2O, can also function as a control rod since it has the ability to absorb neutrons.
Coolants
Now that the heat is obtained from the nuclear reactor, we need something to transfer said heat to water so that it can become steam and rotate the turbines. This is done by the coolant.

Almost all of the currently operating nuclear reactors use water as a coolant because of its high heat capacity. There are some reactors that use liquid sodium as a coolant instead. The advantage of using liquid metals is that they’re a weak moderator compared to water. Hence, for certain reactors, they are preferred.

Current Contribution
The first commercial power plants started in the 1950s. Since then, the number of reactors has risen and the contribution of nuclear energy to power the world is around 10% of the total power consumed.

Although nuclear energy has had a bad rap thanks to its association with weaponry and the multiple tragic accidents, data shows that it is one of the most reliable and safest form of energy production in the world.
Nuclear reactors have the highest capacity factors when compared with the conventional sources of energy.

What this means, is that nuclear power plants are working at maximum power for over 93% of the time. Reasons for this could be because they require less maintenance and are designed to operate for longer stretches before refuelling (typically every 1.5 or 2 years). Considering that a typical nuclear reactor produces 1 gigawatt (GW) of electricity, you cannot simply replace it with 1-gigawatt coal or renewable plant. Based on the capacity factors, you would need almost two coal or three to four renewable plants (each of 1 GW size) to generate the same amount of electricity.
The other factor that many cite for their apprehension toward nuclear energy is the danger associated. But, the truth is that nuclear energy has reduced the amount of greenhouse gas released for producing energy.

This data shows that combating global warming without the help of nuclear energy will be extremely difficult since the gap left by nuclear would be most likely filled overwhelmingly by fossil fuels, thus releasing a lot more greenhouse gases into the atmosphere. This would result in higher rates of global warming. Thus, nuclear energy is greener than what public perception tends to be.
As for the number of deaths, there have been studies conducted that estimate that the number of deaths prevented by using nuclear instead of other conventional sources ranges in the millions.


The Future Outlook…
With improvements in technology, the efficiency and safety of nuclear reactors are only bound to increase. Understandably, there are still concerns about the negatives associated with nuclear reactors. After all, one of the worst man-made disasters of all time was a nuclear reactor accident, and the waste produced is extremely dangerous. Not to mention, the technology involved can be misused for other nefarious purposes.
But we cannot ignore the fact that this source of energy is one of the most reliable, safest and greenest we have as of now. Although not renewable, future research into the use of thorium as a fuel source could mitigate that problem. It is only a matter of time; the world will have to address issues like climate change and the energy needs of an ever-increasing population. Nuclear energy has shown that it has the potential to help ease the crisis as a stepping stone to better, greener energy sources.
The nucleus of an atom was forged in the furnace at the heart of a star. It is that energy endowed by some of the most powerful systems in the universe that we have learned to harvest and use, for better or for worse. It is important to remember that although nuclear energy and nuclear weapons are two sides of the same coin, the former can show us a way to a better future. Better education about the workings, risks and advantages could help advance this technology and improve it. Hopefully, this article has given you some insight to make an informed decision about all things nuclear.

