Firstly, let us know about matter. Matter is basically defined as any substance that has mass and takes up space by having volume.
We all know that matter is made up of atoms which in turn is made up of fundamental particles. These atoms and the molecules they form are held together by a form of potential energy called chemical energy. Then the atoms and molecules also have kinetic energy which accounts for its motion. The changes in these energies of a substance can lead to state change.
Now, let us look into the five well-established states of matter i.e four natural states of matter (solid, liquid, gas and plasma) and one man-made state of matter (Bose-Einstein Condensate)
Index
Solids
Solid is a state of matter wherein the atoms and molecules or packed very tightly and the force between particles is so strong that they can’t move, they can just vibrate. So, in a solid, the atoms have very low kinetic energy.
As the atoms are packed in there, solids have high density and it is very difficult to compress solids. Solids have a definite shape and don’t take the shape of the vessel like liquids or gases.
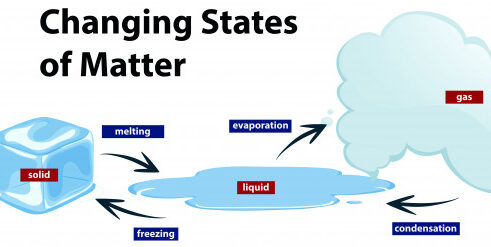
A solid can transform into a liquid through melting, and a liquid can transform into a solid through freezing. A solid can also change directly into a gas through a process called sublimation.
Liquids
In liquids, the particles are loosely packed when compared to solids which give liquids an ability to flow and take the shape of its container. Liquids still have a definite volume and is difficult to compress.
A liquid can be converted to gas through heating at constant pressure to the substance’s boiling point or through the reduction of pressure at a constant temperature. This process of a liquid changing to a gas is called evaporation.
Gases
Gases as we know have a good amount of space between each particle, they have very weak bonds or no bonds at all. That’s why gases expand to fill the container they are put into. Gas molecules have high kinetic energy.
A gas at a temperature below its critical temperature can also be called a vapor. Vapor can be liquefied by compression without cooling. It can also exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid (or solid).
Plasma
Plasma is the most common state of matter in the universe as all the stars are hot balls of plasma.
Plasma is made up of highly charged particles with extremely high kinetic energy. The plasma state is seens when noble gases are used to make glowing signs by using electricity.
Bose-Einstein Condensate
Bose-Einstein Condensate (BEC) is a matter which is formed at extremely low temperatures where there is almost no kinetic energy and thus atoms start to clump together which is called as BEC
This is a man-made state of matter. This state was first predicted, generally, in 1924–1925 by Albert Einstein following and crediting a pioneering paper by Satyendra Nath Bose on the new field now known as quantum statistics.
It was created for the first time in 1995 by Eric Cornell and Carl Weiman, scientists at the Joint Institute for Lab Astrophysics (JILA) in Boulder, Colorado. They cooled a sample of rubidium to within a few degrees of absolute zero using a combination of lasers and magnets.

So, those were the five well established states of matter with a small brief on every state

AWESOME