The Photoelectric effect was a very significant phenomenon in the development of modern physics. It was a part of the age-old puzzle of the nature of light (wave versus particle) which was finally solved by Albert Einstein in 1905 for which he was awarded the Nobel Prize. So, let us get to know about this effect.
What is the Photoelectric Effect?
It is basically a phenomenon wherein a material (generally metals) emits electrically charged particles when electromagnetic radiation like light falls on it. This is the effect that proved the particle nature of light.
Who discovered the Photoelectric Effect?
The photoelectric effect was discovered by the German physicist Heinrich Rudolf Hertz in 1887. Hertz, while working on radio waves, observed that, when ultraviolet light hits two metal electrodes with a voltage applied across them, the light changes the voltage at which sparking takes place. This relation between light and electricity, thus photoelectric, was clarified in 1902 by another German physicist, Philipp Lenard.
Let us see and understand three major observations of this phenomenon which caught the interest of physicists:
- The maximum kinetic energy of the emitted electrons did not change with intensity and this observation goes against the classical wave nature of light.
- With the increase in the intensity of light, the number of emitted electrons increased which puzzled physicists.
- The third important observation on the photoelectric effect was that the metal doesn’t emit electrons for all kinds of lights.
The observations clearly showed that light should have particles with fixed energy which depends on its frequency. Thus, Albert Einstein formed a new theory of light which stated that each particle of light called photon has a fixed amount of energy which depends on its frequency. With an increase in the intensity of light, the number of photons increases eventually increasing the number of emitted electrons.
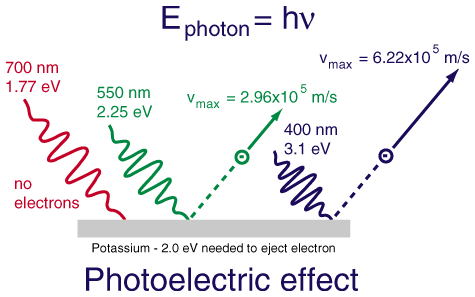
Let us see the photoelectric equation and its terms to understand the third observation.
The Photoelectric Equation
KEphotoelectron = Ephoton – Eo
Ephoton = Energy of the photon
KEphotoelectron = Kinetic Energy of the emitted electron
Eo = Work Function of the metal (It is the minimum energy needed to knock off an electron and is different for different metals)
Ephoton = hc/Λ
Where,
h = planck’s constant = 6.62607015 × 10−34 joule second
c = speed of light = 3×108 ms-1
Λ = Wavelenght
So, only when Ephoton is greater then Eo an electron is emitted.
So, I hope you got an overview of the photoelectric effect, how it leads to particle behavior, and how did physicists explain the observations.
Let us see some of the applications of this phenomenon…
Photoelectric Effect – Applications
Though the photoelectric effect sounds like some random theoretical phenomenon, it have a lot of real life applications.
- Photocell: Photocells are commonly found in solar panels. It works on the simple process of the light striking the cathode which causes the emission of electrons converting heat light into electricity.
- Scintillators: A scintillator is a device that emits light when it attracts radiation from either source in a lab or cosmic sources. Scintillators are used in particle detectors, new energy resource exploration, X-ray security, nuclear cameras, computed tomography, and gas exploration.
- Photomultiplier: These tubes make use of photoelectric effect to convert light intensity into electrical currents.
- The photoelectric effect also finds its applications in photocopies, light meter, photodiodes, phototransistors.
So, those were few of the application wherein the photoelectric effect is found. I hope you got an overview of this amazing phenomenon. Do share it with your science enthusiast friends.

Pingback: Quantum Theory - A Theory Which Completely Changed Our Understanding