Millikan Oil Drop Experiment is one of the most popular experiments as it was the first-ever experiment that gave us the direct measurement of the charge of a single electron.
As we all know, J.J Thomson discovered electron for the first time in 1897 with his cathode ray tube (CRT) experiment. He also found the charge to mass ratio (e/me) of an electron as 1.758820 × 1011 CKg-1
In 1909, Robert A. Millikan devised this method known as the oil drop experiment to find out the charge of an electron. He succeeded in it and found the charge to be -1.6 × 10-19 C.
So, let us get to know about the experiment now.
Experiment
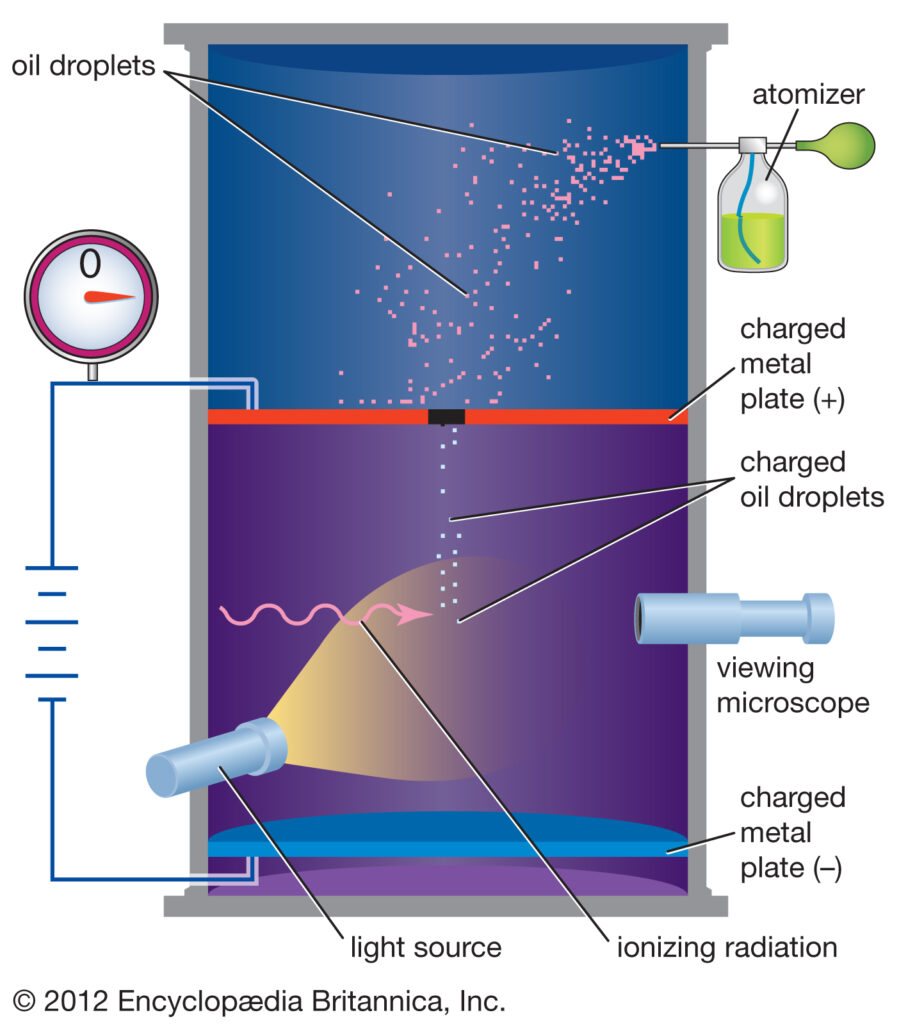
In this experiment, oil droplets in the form of mist, produced by the atomizer were allowed to enter through a tiny hole in the upper plate of the electrical condenser as you can see in the image.
The downward motion of these droplets was viewed through the telescope, equipped with a micrometer eyepiece. By measuring the rate of fall of these droplets, Millikan was able to measure the mass of oil droplets (m). The air inside the chamber was ionized by passing X-rays through it.
The oil drops gained negative charge due to the ionized gases inside the chamber. The fall of these oil droplets can be slowed, accelerated, or made stationary depending upon the charge on the droplets and the voltage applied to the plate. The amount of voltage needed to make the droplets stationary (i.e electrical force = gravitational force) was used along with its mass to determine the overall electric charge on the droplet
By carefully measuring the effects of electrical field strength on the motion of oil droplets, Millikan concluded that the magnitude of electrical charge, q, on the droplets is always an integral multiple of the electrical charge, e (charge of an electron), that is, q = n × e, where n = 1, 2, 3…
He found the value of e to be -1.6 × 10-19 C which was very close to the present value of -1.602 × 10-19 C. Even after that a lot of proofs came up saying that the total charge is always a whole number multiple of a constant value which is the charge of an electron (e).
The mass of the electron (me) was determined by combining the result of the Oil Drop Experiment with Thomson’s value of the (e/me) ratio.
e/(e/me) = (1.6 × 10-19 C) / (1.758820 × 1011 CKg-1)
me = 9.1094 × 10-31kg
So, that was an overview of the Millikan Oil Drop Experiment. Hope you got a basic overview of this experiment.
FAQ
Millikan’s oil drop experiment determined the charge of a single electron and thus helped in finding the mass of an electron as well.
Droplets of oil are used instead of water, to reduce the tendency of the droplets to evaporate while the experiment is being performed
The experiment will not work in vacuum as it needs ionized gas for the oil droplets to get charged.