Soon after the discovery of fundamental particles, a lot of scientists tried to explain the distribution of these particles inside the atom with an atomic model. One of such models is Rutherford’s Atomic Model.
The first such atomic model was the plum pudding model by J. J. Thomson. According to Thomson’s model, an atom is like a sphere with a radius of approximately 10-10 and the positive charge is uniformly distributed across it. It said that electrons are embedded in the most stable configuration across the positive charge distribution. Thomson failed to explain the experiments done by Rutherford and thus came the Rutherford’s Atomic Model.
According to Rutherford’s model, the positively charged particles and most of the mass of an atom was concentrated in an extremely small volume. He called this region as a nucleus. Rutherford’s model proposed that the negatively charged electrons moved the nucleus in circular orbits.
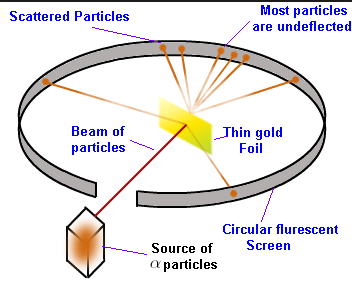
Rutherford proposed his atomic model based on his Alpha Ray Scattering Experiment, so, let us understand the experiment and its observations
Index
Alpha Ray Scattering Experiment
In this experiment, Rutherford directed high energy α-particles from a radioactive source onto a thin (100nm thickness) gold foil. The α-particles after passing through gold foil hit a zinc sulfide screen which then gives a small flash of light
α-particle: Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. Generally, alpha decay produces these particles.
Rutherford got some observation and conclusions from this experiment which proved Thomson’s Model completely wrong, those were
- A huge fraction of total α-particles passed through the gold sheet without any deflection which leads to the conclusion that most of the space inside an atom is empty.
- A few α-particles deflected slightly. This gave a conclusion that positive charge is not uniformly distributed and is concentrated in a small volume.
- A very few α-particles (1 in 20,000) deflected back which means that only a few α-particles had an angle of deflection close to 180-degree. So, the volume occupied by the positively charged particles in an atom is very small as compared to the total volume of an atom.
Based on these conclusions, Rutherford proposed the so-called Nuclear or Planetary atomic model in 1911.
Rutherford’s Atomic Model
This atomic model described an atom in the following way
- The positive charge is concentrated in a very small volume at the centre of the atom. Most of the mass of an atom is in this small volume. Rutherford called it nucleus.
- The negatively charged particles i.e electrons revolve around the nucleus with very high speed in circular paths. Rutherford called the circular paths, orbits.
- Electrostatic forces hold electrons and nucleus together.
This model told us a lot of correct things about the distribution of fundamental particles inside an atom. But as a coin have two sides, the model failed to explain a few things. So, let us look at the drawbacks of this model.
Drawbacks
A few major drawbacks of the Rutherford’s model are:
- According to the model, electrons revolve around the nucleus in circular orbits. But we know that a body moving in orbit must accelerate, thus, electrons should accelerate.
According to Maxwell’s theory, a charged accelerating particle must emit electromagnetic radiation. If this happens with electrons, the orbit would eventually shrink and electrons would fall into nucleus which doesn’t happen. So, this is a contradiction to this model. - Another major drawback of this model is that it didn’t tell anything about the exact arrangement of electrons around the nucleus and the energy of these electrons.
The next atomic model, namely Bohr’s atomic model, addressed all these drawbacks.
So, that was a glimpse of Rutherford’s Atomic Model. Put your doubt in the comments below, we will surely try to clarify them.
FAQ
Rutherford’s atomic model basically states that all the positive charge and most of the mass is concentrated in a very small volume at the centre. The negative charge i.e electrons go around the positive charge with very high speed in circular paths. Electrostatic forces hold electrons and nucleus together
Rutherford’s model was proposed in 1911.

Pingback: Bohr's Atomic Model | AtomsTalk